Patents by Inventor Anthony S. Fauci - Koon Yew Yin
Koon Yew Yin
Publish date: Sun, 23 May 2021, 05:45 PM
The video interview of Dr Judy Mikovits has gone viral.
After I have seen this video namely Fact-checking Judy Mikovits, the controversial virologist attacking Anthony Fauci in a viral conspiracy video by Matin Enserink, Jon Cohen, May 8 2020, I doubted its accuracy. If it is true, Dr Fauci is a multi-billionaire.
So, I Googled “Patents by Anthony S. Fauci” and I was shocked to see the following:
Anthony S. Fauci has filed for patents to protect the following inventions. This listing includes patent applications that are pending as well as patents that have already been granted by the United States Patent and Trademark Office (USPTO).
- Use of antagonists of the interaction between HIV GP120 and ?4?7 integrin
Patent number: 9896509
Abstract: Methods are provided for the treatment of a HIV infection. The methods can include administering to a subject with an HIV infection a therapeutically effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist, thereby treating the HIV infection. In several examples, the ?4 integrin antagonist is a monoclonal antibody that specifically binds to a ?4, ?1 or ?7 integrin subunit or a cyclic hexapeptide with the amino acid sequence of CWLDVC. Methods are also provided to reduce HIV replication or infection. The methods include contacting a cell with an effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist. Moreover, methods are provided for determining if an agent is useful to treat HIV.
Type: Grant
Filed: August 3, 2016
Date of Patent: February 20, 2018
Assignee: The United States of America, as Represented by the Secretary, Department of Health and Human Services
Inventors: James Arthos, Diana Goode, Claudia Cicala, Anthony S. Fauci
- USE OF ANTAGONISTS OF THE INTERACTION BETWEEN HIV GP120 AND A4B7 INTEGRIN
Publication number: 20160333097
Abstract: Methods are provided for the treatment of a HIV infection. The methods can include administering to a subject with an HIV infection a therapeutically effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist, thereby treating the HIV infection. In several examples, the ?4 integrin antagonist is a monoclonal antibody that specifically binds to a ?4, ?1 or ?7 integrin subunit or a cyclic hexapeptide with the amino acid sequence of CWLDVC. Methods are also provided to reduce HIV replication or infection. The methods include contacting a cell with an effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist. Moreover, methods are provided for determining if an agent is useful to treat HIV.
Type: Application
Filed: August 3, 2016
Publication date: November 17, 2016
Applicant: THE UNITED STATES OF AMERICA, as represented by the Secretary, Department of Health and Human Serv
Inventors: James Arthos, Diana Goode, Claudia Cicala, Anthony S. Fauci
- Use of antagonists of the interaction between HIV GP120 and ?4?7 integrin
Patent number: 9441041
Abstract: Methods are provided for the treatment of a HIV infection. The methods can include administering to a subject with an HIV infection a therapeutically effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist, thereby treating the HIV infection. In several examples, the ?4 integrin antagonist is a monoclonal antibody that specifically binds to a ?4, ?1 or ?7 integrin subunit or a cyclic hexapeptide with the amino acid sequence of CWLDVC. Methods are also provided to reduce HIV replication or infection. The methods include contacting a cell with an effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist. Moreover, methods are provided for determining if an agent is useful to treat HIV.
Type: Grant
Filed: September 21, 2015
Date of Patent: September 13, 2016
Assignee: The United States of America, as Represented by the Secretary, Department of Health and Human Services
Inventors: James Arthos, Diana Goode, Claudia Cicala, Anthony S. Fauci
- USE OF ANTAGONISTS OF THE INTERACTION BETWEEN HIV GP120 AND A4B7 INTEGRIN
Publication number: 20160075786
Abstract: Methods are provided for the treatment of a HIV infection. The methods can include administering to a subject with an HIV infection a therapeutically effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist, thereby treating the HIV infection. In several examples, the ?4 integrin antagonist is a monoclonal antibody that specifically binds to a ?4, ?1 or ?7 integrin subunit or a cyclic hexapeptide with the amino acid sequence of CWLDVC. Methods are also provided to reduce HIV replication or infection. The methods include contacting a cell with an effective amount of an agent that interferes with the interaction of gp120 and ?4 integrin, such as a ?4?1 or ?4?7 integrin antagonist. Moreover, methods are provided for determining if an agent is useful to treat HIV.
Type: Application
Filed: September 21, 2015
Publication date: March 17, 2016
Applicant: The United States of America, as Represented by the Secretary, Department of Health and Human Serv
Inventors: James Arthos, Diana Goode, Claudia Cicala, Anthony S. Fauci
- IMMUNOCONJUGATES COMPRISING CD4 AND IMMUNOGLOBIN MOLECULES FOR THE TREATMENT OF HIV INFECTION
Publication number: 20090285815
Abstract: Nucleic acids encoding recombinant CD4-fusion proteins are disclosed herein that include a CD4 polypeptide ligated at its C-terminus with a portion of an immunoglobulin comprising a hinge region and a constant domain of a mammalian immunoglobulin heavy chain. The portion of the IgG is fused at its C-terminus with a polypeptide comprising a tailpiece from the C terminus of the heavy chain of an IgA antibody or a tailpiece from a C terminus of the heavy chain of an IgM antibody. Also disclosed herein are methods for using these CD4-fusion proteins.
Type: Application
Filed: March 21, 2008
Publication date: November 19, 2009
Inventors: James Arthos, Claudia Cicala, Anthony S. Fauci
- Fusion protein including of CD4
Patent number: 7368114
Abstract: Novel recombinant polypeptides are disclosed herein that include a CD4 polypeptide ligated at its C-terminus with a portion of an immunoglobulin comprising a hinge region and a constant domain of a mammalian immunoglobulin heavy chain. The portion or the IgG is fused at its C-terminus with a polypeptide comprising a tailpiece from the C-terminus of the heavy chain of an IgA antibody ara tailpiece from a C-terminus of the heavy chain of an IgM antibody. Also disclosed herein are methods for using these CD4 fusion proteins.
Type: Grant
Filed: October 24, 2002
Date of Patent: May 6, 2008
Assignee: The United States of America as represented by the Department of Health and Human Services
Inventors: James Arthos, Claudia Cicala, Anthony S. Fauci
- HIV related peptides
Patent number: 6911527
Abstract: This invention is the discovery of novel specific epitopes and antibodies associated with long term survival of HIV-1 infections. These epitopes and antibodies have use in preparing vaccines for preventing HIV-1 infection or for controlling progression to AIDS.
Type: Grant
Filed: January 7, 2000
Date of Patent: June 28, 2005
Assignee: The United States of America as represented by the Secretary of the Department of Health and Human Services
Inventors: Giuseppe Scala, Xueni Chen, Oren J. Cohen, Anthony S. Fauci
- Efficient inhibition of hiv-1 viral entry through a novel fusion protein including of cd4
Publication number: 20040265306
Abstract: Novel recombinant polypeptides are disclosed herein that include a CD4 polypeptide ligated at its C-terminus with a portion of an immunoglobulin comprising a hinge region and a constant domain of a mammalian immunoglobulin heavy chain. The portion of the IgG is fused at its C-terminus with a polypeptide comprising a tailpiece from the C-terminus of the heavy chain of an IgA antibody or a tailpiece from a C-terminus of the heavy chain of an IgM antibody. Also disclosed herein are methods for using these CD4-fusion proteins.
Type: Application
Filed: July 27, 2004
Publication date: December 30, 2004
Inventors: James Arthos, Claudia Cicala, Anthony S. Fauci
- Immunologic enhancement with intermittent interleukin-2 therapy
Publication number: 20030180254
Abstract: A method for activating a mammalian immune system entails a series of IL-2 administrations that are effected intermittently over an extended period. Each administration of IL-2 is sufficient to allow spontaneous DNA synthesis in peripheral blood or lymph node cells of the patient to increase and peak, and each subsequent administration follows the preceding administration in the series by a period of time that is sufficient to allow IL-2 receptor expression in peripheral or lymph node blood of the patient to increase, peak and then decrease to 50% of peak value. This intermittent IL-2 therapy can be combined with another therapy which targets a specific disease state, such as an anti-retroviral therapy comprising, for example, the administration of AZT, ddI or interferon alpha. In addition, IL-2 administration can be employed to facilitate in situ transduction of T cells in the context of gene therapy.
Type: Application
Filed: January 23, 2003
Publication date: September 25, 2003
Applicant: The Govt. of the USA as represented by the Secretary of the Dept. of Health & Human Services
Inventors: H. Clifford Lane, Joseph A. Kovacs, Anthony S. Fauci
- Immunologic enhancement with intermittent interleukin-2 therapy
Patent number: 6548055
Abstract: A method for activating a mammalian immune system entails a series of IL-2 administrations that are effected intermittently over an extended period. Each administration of IL-2 is sufficient to allow spontaneous DNA synthesis in peripheral blood or lymph node cells of the patient to increase and peak, and each subsequent administration follows the preceding administration in the series by a period of time that is sufficient to allow IL-2 receptor expression in peripheral or lymph node blood of the patient to increase, peak and then decrease to 50% of peak value. This intermittent IL-2 therapy can be combined with another therapy which targets a specific disease state, such as an anti-retroviral therapy comprising, for example, the administration of AZT, ddI or interferon alpha. In addition, IL-2 administration can be employed to facilitate in situ transduction of T cells in the context of gene therapy.
Type: Grant
Filed: August 9, 2000
Date of Patent: April 15, 2003
Assignee: The United States of America as represented by the Department of Health and Human Services
Inventors: H. Clifford Lane, Joseph A. Kovacs, Anthony S. Fauci
- Immunologic enhancement with intermittent interleukin-2 therapy
Patent number: 6190656
Abstract: A method for activating a mammalian immune system entails a series of IL-2 administrations that are effected intermittently over an extended period. Each administration of IL-2 is sufficient to allow spontaneous DNA synthesis in peripheral blood or lymph node cells of the patient to increase and peak, and each subsequent administration follows the preceding administration in the series by a period of time that is sufficient to allow IL-2 receptor expression in peripheral or lymph node blood of the patient to increase, peak and then decrease to 50% of peak value. This intermittent IL-2 therapy can be combined with another therapy which targets a specific disease state, such as an anti-retroviral therapy comprising, for example, the administration of AZT, ddI or interferon alpha. In addition, IL-2 administration can be employed to facilitate in situ transduction of T cells in the context of gene therapy.
Type: Grant
Filed: September 2, 1997
Date of Patent: February 20, 2001
Assignee: The United States of America as represented by the Department of Health and Human Services
Inventors: H. Clifford Lane, Joseph A. Kovacs, Anthony S. Fauci
- Immunologic enhancement with intermittent interleukin-2 therapy
Patent number: 5696079
Abstract: A method for activating a mammalian immune system entails a series of IL-2 administrations that are effected intermittently over an extended period. Each administration of IL-2 is sufficient to allow spontaneous DNA synthesis in peripheral blood or lymph node cells of the patient to increase and peak, and each subsequent administration follows the preceding administration in the series by a period of time that is sufficient to allow IL-2 receptor expression in peripheral or lymph node blood of the patient to increase, peak and then decrease to 50% of peak value. This intermittent IL-2 therapy can be combined with another therapy which targets a specific disease state, such as an anti-retroviral therapy comprising, for example, the administration of AZT, ddI or interferon alpha. In addition, IL-2 administration can be employed to facilitate in situ transduction of T cells in the context of gene therapy.
Type: Grant
Filed: May 26, 1995
Date of Patent: December 9, 1997
Assignee: The United States of America as represented by the Department of Health and Human Services
Inventors: H. Clifford Lane, Joseph A. Kovacs, Anthony S. Fauci
More articles on Koon Yew Yin's Blog
Created by Koon Yew Yin | Jun 28, 2024

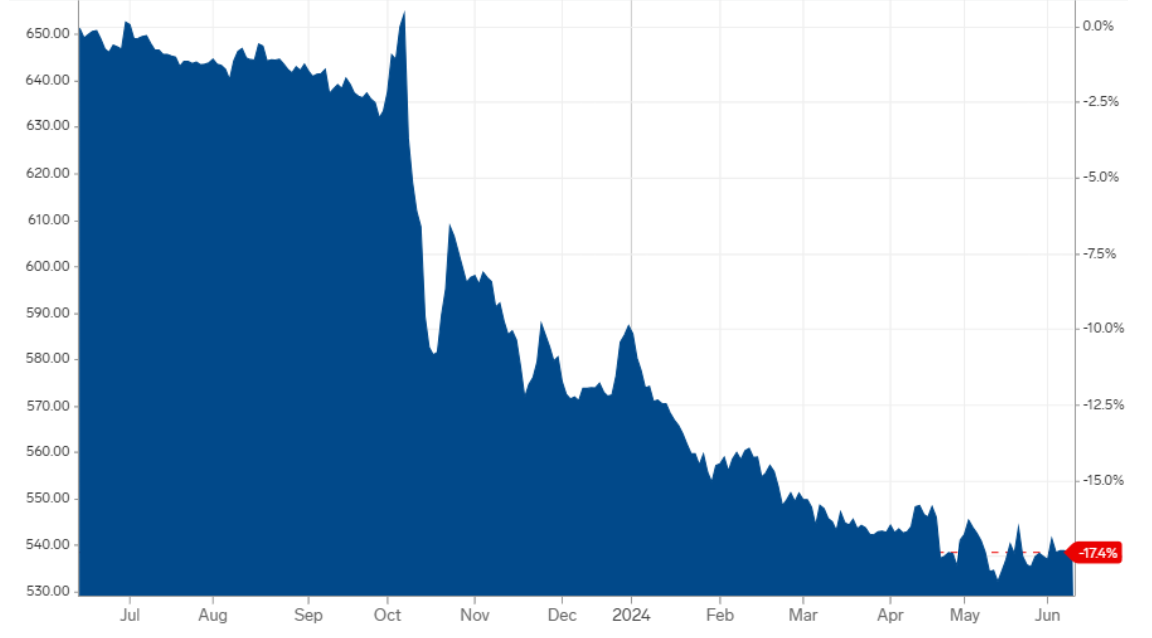
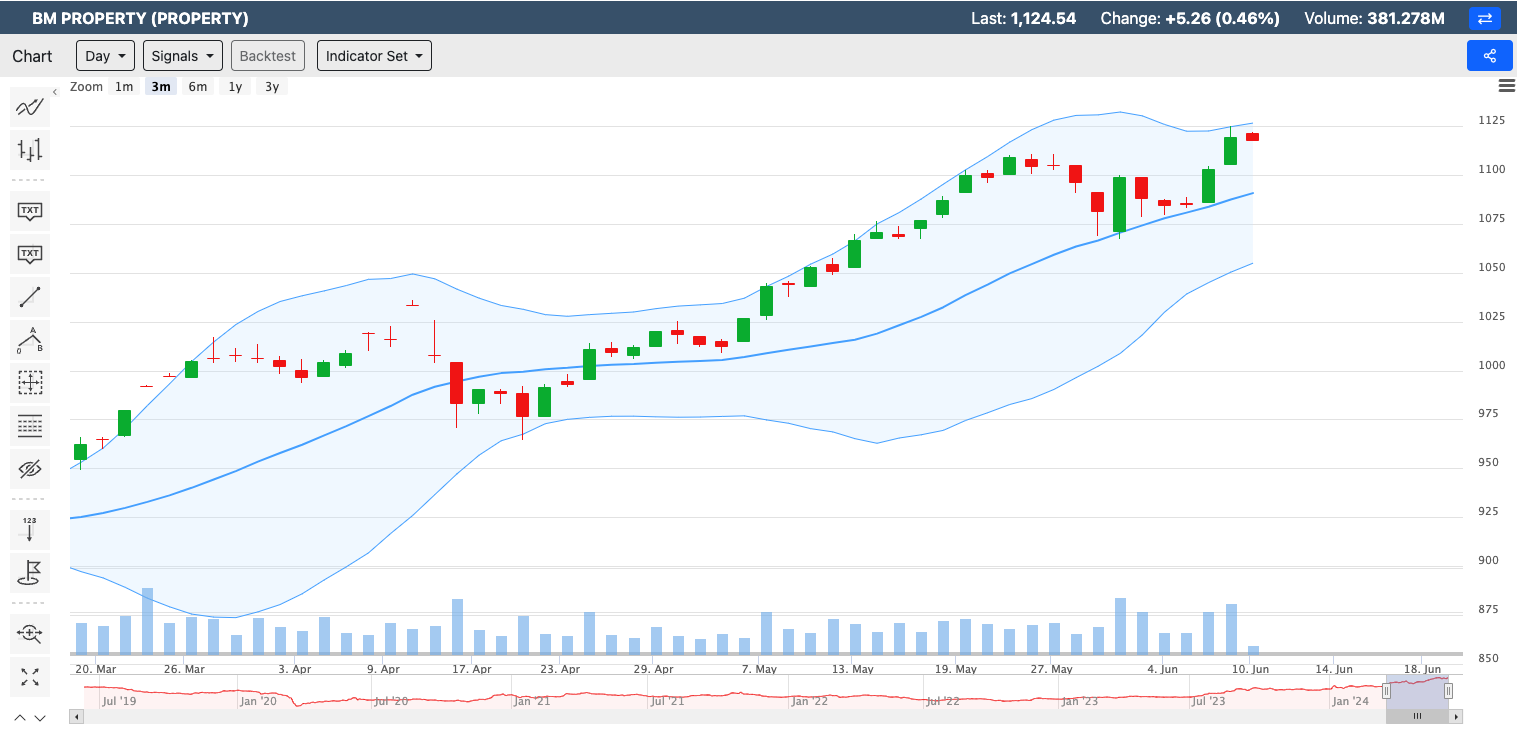


Created by Koon Yew Yin | May 28, 2024
It is a human nature that most men would complain to their wives that they were overworked and the wives would tell their husbands to get some assistants. As a result, the number of staff increases...
Created by Koon Yew Yin | May 13, 2024
Eversendai Corporation Berhad recently reported its earnings results for the fourth quarter ended December 31, 2023. Here are the key financial highlights:
Created by Koon Yew Yin | May 06, 2024
Eversendai Corporation Berhad made a remarkable comeback in FY2023, reporting strong profit growth. Here are the key highlights from their financial performance:
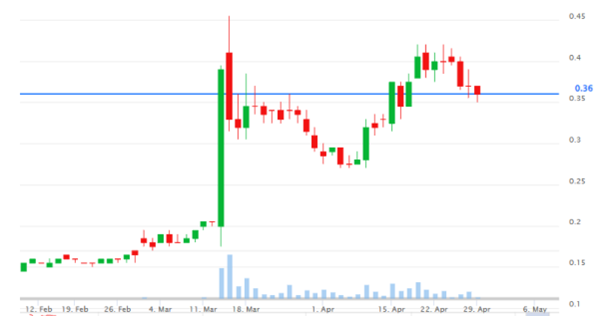
Created by Koon Yew Yin | Apr 30, 2024
As shown on the chart below, Sendai has been dropping in the last few days. Today all shareholders must be wondering to sell, hold on or to buy some at a cheaper price.














