Is HARTA AMG GLOVE obtaining FDA approval?
gloveharicut
Publish date: Tue, 28 Jul 2020, 01:59 PM
{{{PM me to join GLOVE Private Discussion Room}}}
I invite you to read my blog and make a smart GLOVE decision.
https://klse.i3investor.com/blogs/gloveharicut/blidx.jsp
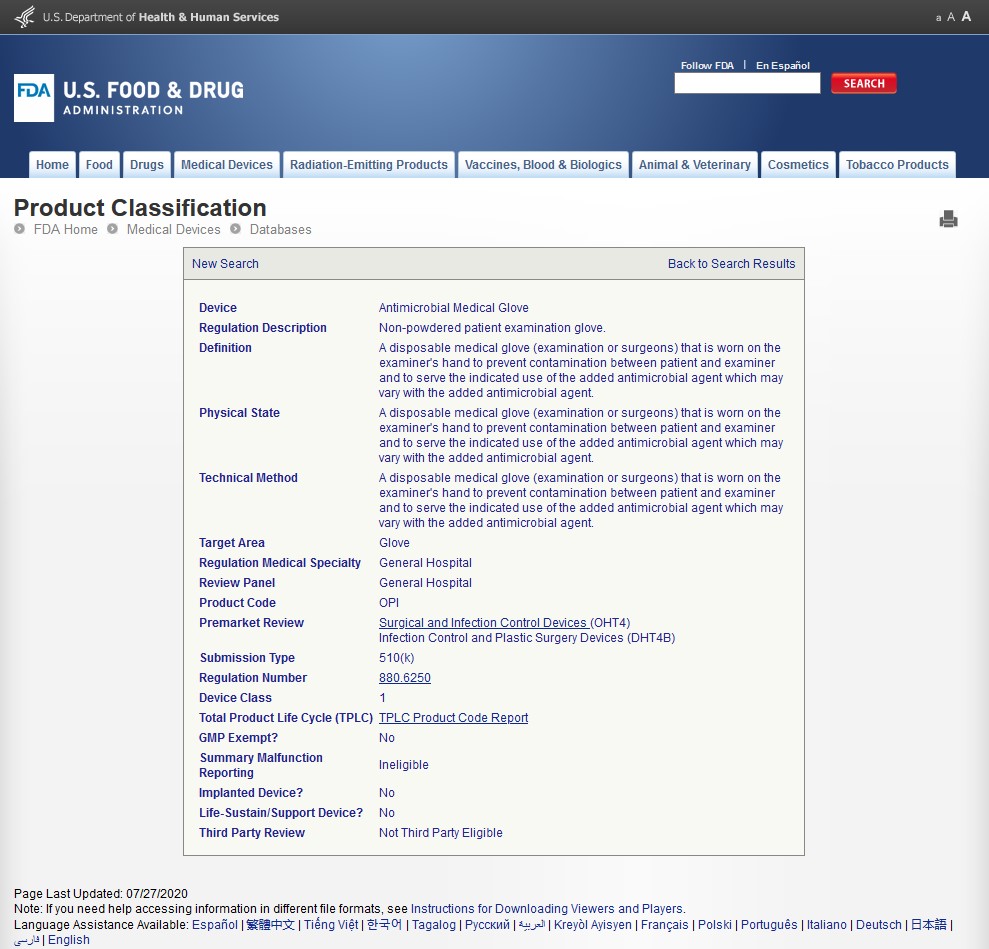
Is HARTA AMG obtaining FDA approval?
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=2766
Related Stocks
| Chart | Stock Name | Last | Change | Volume |
|---|
More articles on gloveharicut
Created by gloveharicut | Sep 20, 2022
Created by gloveharicut | Aug 30, 2021
Created by gloveharicut | May 04, 2021
Created by gloveharicut | Apr 19, 2021
Discussions
I think this is submission only. If approves, it will show in 510(k) Clearances.
Overview
Section 510(k) of the Food, Drug and Cosmetic Act requires device manufacturers who must register, to notify FDA of their intent to market a medical device at least 90 days in advance. This is known as Premarket Notification - also called PMN or 510(k). This allows FDA to determine whether the device is equivalent to a device already placed into one of the three classification categories. Thus, "new" devices (not in commercial distribution prior to May 28, 1976) that have not been classified can be properly identified. Specifically, medical device manufacturers are required to submit a premarket notification if they intend to introduce a device into commercial distribution for the first time or reintroduce a device that will be significantly changed or modified to the extent that its safety or effectiveness could be affected. Such change or modification could relate to the design, material, chemical composition, energy source, manufacturing process, or intended use.
2020-07-28 17:44






















Aero1
Think so
2020-07-28 14:17